I've Been Implementing AI in Marketing Since 2012. Here's What Actually Works in Pharma
In 2012, I deployed IBM Watson to analyze petition comments for a global advocacy organization, extracting sentiment patterns that transformed their email engagement rates. Most marketers were still debating whether social media was a fad.
Thirteen years later, I'm watching pharmaceutical companies make the same fundamental mistake with AI that tech companies made with mobile in 2007: treating revolutionary technology like an incremental improvement.
The problem isn't that pharma companies don't understand AI. The problem is they're approaching it exactly like they approach everything else: clinical trials.

Why Pharma's Clinical Trial Mindset Kills AI Implementation
Most pharmaceutical companies approach AI like they approach drug development: hypothesis, small-scale testing, gradual expansion. This methodical approach works brilliantly for bringing life-saving medications to market. It fails catastrophically for AI implementation.
Why? Because AI isn't a drug that needs safety validation. It's a business tool that needs performance validation.
When you treat AI like a clinical trial, you get:
- Six-month "pilot programs" testing obvious use cases
- Committees evaluating AI tools the same way they evaluate new compounds
- Risk frameworks designed for patient safety applied to marketing automation
- Success metrics borrowed from regulatory approval processes
The result? By the time your AI "trial" concludes, the technology has evolved three generations and your competitors have implemented solutions you're still testing.
The Watson Reality Check: What I Learned in 2012 Still Applies
When I first implemented Watson for sentiment analysis, I wasn't running a pilot program. I was solving a specific business problem: a global advocacy group needed to understand what motivated their petition signers so they could craft more effective email campaigns.
Watson analyzed thousands of user comments, identified language patterns that indicated high engagement, and enabled the team to mirror successful messaging strategies. The result wasn't a statistical improvement—it was a fundamental shift in how they approached campaign messaging.
Here's what that taught me about AI implementation that still holds true:
- Start with the business problem, not the technology. The most successful AI implementations I've seen in pharma start with a clear performance gap: "Our HCP engagement emails have a 3% click rate, but competitor intelligence suggests 8% is achievable." Then you find the AI solution that closes that gap.
- Deploy for performance, not experimentation. Every AI tool I've implemented since Watson has been deployed to improve an existing process, not to "explore AI capabilities." When Velomacchi needed to understand customer preferences in the premium luggage market, I used Watson to analyze Reddit discussions in r/backpacks. The goal wasn't to test AI—it was to replace expensive focus groups with real-time customer intelligence.
- Scale quickly or don't start. The companies that succeed with AI treat it like marketing automation in 2010: deploy, measure, optimize, expand. The companies that fail treat it like a new therapeutic area: study, committee review, limited deployment, extensive evaluation.
What Actually Works: Real Pharma AI Use Cases
Based on thirteen years of implementation experience across multiple industries, including my recent work at Novartis, here are the AI applications that deliver measurable ROI in pharmaceutical marketing:
Content Personalization at Scale
The most successful implementation I've seen involved a top-10 pharma company using AI to personalize HCP communications across 47 countries. Instead of running a six-month pilot, they deployed AI content generation for three therapeutic areas simultaneously.
The AI analyzed successful email campaigns, identified messaging patterns that drove engagement, and generated personalized content variations for different HCP segments. Within 90 days, they saw a 34% improvement in email engagement rates.
Why it worked: They treated AI like marketing automation, not like a new drug candidate.
Regulatory Content Compliance
At a biotech, we integrated AI for real-time compliance checking across our global web platform. The AI system reviewed content changes against regulatory requirements in 11 countries, flagging potential issues before publication.
This wasn't a pilot program—it was a business necessity. When you're managing 15 websites across different regulatory environments, manual compliance review creates weeks of delays. AI compliance checking reduced review time from 5-7 days to 2-3 hours.
Competitive Intelligence Automation
One of the most underutilized AI applications in pharma is automated competitive intelligence. I've implemented systems that monitor competitor websites, regulatory filings, and scientific publications for strategic insights.
The AI identifies pattern changes in competitor messaging, tracks new product positioning, and alerts teams to potential market shifts. This provides strategic intelligence that would require dedicated analyst teams to gather manually.
Medical Affairs Content Generation
Medical affairs teams spend enormous amounts of time creating scientific content for different audiences. AI content generation can transform this process by:
- Converting clinical trial data into HCP-appropriate summaries
- Generating patient education materials from complex scientific content
- Creating regulatory submission documents from research data
- Adapting scientific content for different regional requirements
The key is treating AI as a content production tool, not a content strategy tool. AI excels at format conversion and audience adaptation—but strategic messaging still requires human expertise.
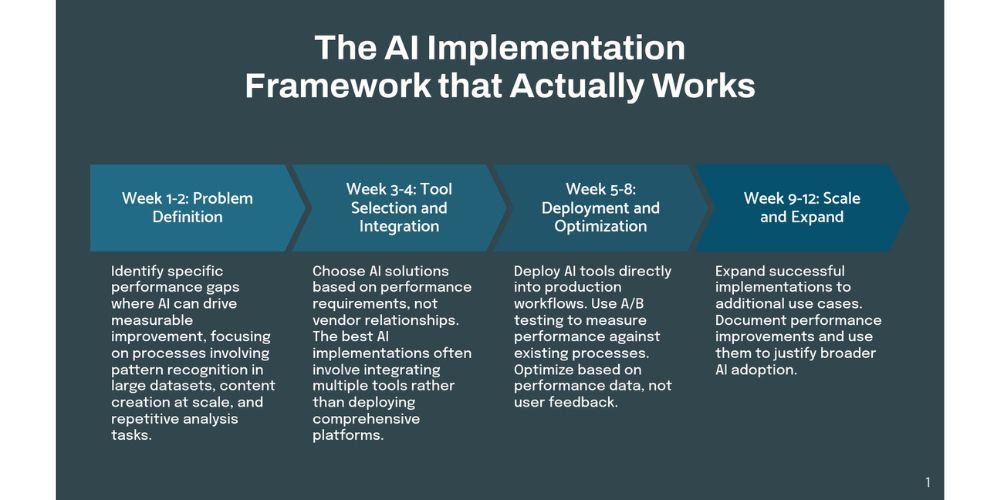
The Implementation Framework That Actually Works
After implementing AI across healthcare, financial services, and technology companies, here's the framework that consistently delivers results:
Week 1-2: Problem Definition
Identify specific performance gaps where AI can drive measurable improvement. Focus on processes that involve:
- Pattern recognition in large datasets
- Content creation at scale
- Repetitive analysis tasks
- Multi-variable optimization
Week 3-4: Tool Selection and Integration
Choose AI solutions based on performance requirements, not vendor relationships. The best AI implementations often involve integrating multiple tools rather than deploying comprehensive platforms.
Week 5-8: Deployment and Optimization
Deploy AI tools directly into production workflows. Use A/B testing to measure performance against existing processes. Optimize based on performance data, not user feedback.
Week 9-12: Scale and Expand
Expand successful implementations to additional use cases. Document performance improvements and use them to justify broader AI adoption.
Notice what's missing from this framework? Committee reviews, pilot programs, and gradual rollouts. When you treat AI like a business tool instead of a clinical trial, implementation moves from months to weeks.
The Regulatory Reality: Why Compliance Isn't the Barrier You Think
Pharmaceutical companies often cite regulatory compliance as the primary barrier to AI adoption. This reflects a fundamental misunderstanding of both regulation and AI implementation.
Most AI marketing applications don't directly impact regulated claims or patient interactions. AI content generation, competitive intelligence, and workflow automation operate in the same regulatory environment as existing marketing tools.
The compliance focus should be on data handling, not AI capabilities. When we implemented AI compliance checking at a biotech, the regulatory review focused on:
- Data privacy and security protocols
- Audit trail requirements for AI-generated content
- Human oversight requirements for customer-facing communications
- Documentation standards for AI decision-making processes
These are standard enterprise software requirements, not unique AI challenges.
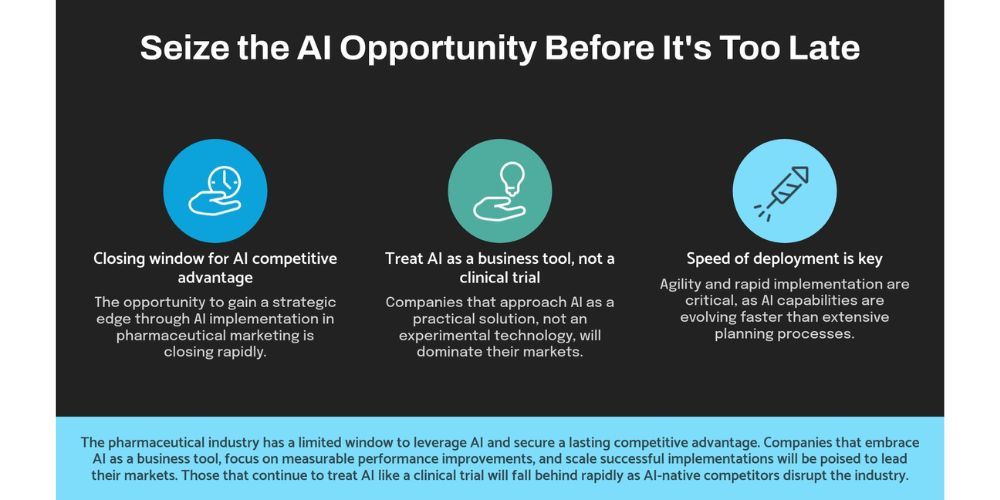
What's Coming Next: The Strategic Implications
Based on current AI development trends and pharmaceutical market dynamics, here are the strategic shifts I'm tracking:
AI-native competitor entry: Technology companies with strong AI capabilities are entering pharmaceutical marketing. When these companies deploy AI-first strategies, traditional pharmaceutical marketing approaches will look as outdated as print advertising.
Real-time regulatory adaptation: AI systems that can adapt content and messaging in real-time based on regulatory changes will become competitive advantages. Companies that treat AI implementation like clinical trials won't have the organizational agility to deploy these systems.
Automated scientific communication: AI will transform how pharmaceutical companies communicate scientific information to healthcare providers. Companies with AI-enhanced medical affairs capabilities will dominate educational content and thought leadership.
Predictive market intelligence: AI analysis of scientific publications, regulatory filings, and market data will enable pharmaceutical companies to predict competitor strategies and market changes. This intelligence advantage will be decisive in therapeutic areas with multiple competing treatments.
The Workshop Preview: Hands-On AI Strategy Development
These insights form the foundation of the AI Strategy Workshop I'm developing for pharmaceutical marketing leaders. The workshop focuses on practical implementation strategies, not theoretical AI capabilities.
Participants develop AI implementation roadmaps for their specific business challenges, using the performance validation framework rather than clinical trial methodologies. We cover tool selection, integration strategies, and performance measurement approaches that deliver results in pharmaceutical environments.
The workshop includes case studies from my AI consulting experience, competitive intelligence implementation guides, and regulatory compliance frameworks that accelerate rather than delay AI adoption.
The Bottom Line: Speed Wins
After thirteen years of AI implementation, from Watson to ChatGPT, one principle consistently determines success: speed of deployment beats perfection of planning.
Pharmaceutical companies have an enormous opportunity to gain competitive advantage through AI implementation. But that opportunity requires treating AI like the business tool it is, not like the drug candidates that built your company.
The companies that win will be those that deploy AI solutions quickly, measure performance rigorously, and scale successful implementations aggressively. The companies that lose will be those still running pilot programs while their competitors transform entire market categories.
The choice is yours. But the window for strategic advantage is closing faster than most pharmaceutical executives realize.
Ready to develop an AI implementation strategy for your pharmaceutical marketing organization? Contact me to discuss the AI Strategy Workshop and how these frameworks can accelerate your competitive positioning.
How do I convince leadership that AI implementation doesn't need a year-long pilot program?
Start with cost comparison, not technology comparison. Show leadership what competitors accomplish with AI in 90 days versus what your current pilot timeline delivers in 18 months. Frame it as competitive intelligence: "While we're piloting email subject line optimization, competitors are deploying AI for real-time regulatory compliance across 90 countries." Use specific ROI examples—34% email engagement improvements, 5-day to 3-hour compliance reviews—to demonstrate that performance validation happens in weeks, not quarters.
What's the biggest regulatory compliance risk with rapid AI deployment in pharma marketing?
The biggest risk isn't AI-specific—it's the same data handling and audit trail requirements you face with any marketing technology. Most pharmaceutical companies overestimate AI regulatory complexity because they confuse patient-facing clinical applications with marketing automation tools. Focus your compliance review on standard enterprise software requirements: data privacy protocols, human oversight for customer communications, and documentation standards. These reviews take weeks, not months, when you treat AI like business software instead of medical devices.
Which AI use cases should pharmaceutical companies prioritize for immediate ROI?
Prioritize AI applications that replace time-intensive manual processes with measurable performance gaps. Content personalization at scale delivers the fastest ROI—AI can generate personalized HCP communications across multiple countries and therapeutic areas in hours instead of weeks. Regulatory compliance checking provides immediate cost savings by reducing review cycles from days to hours. Competitive intelligence automation offers strategic advantages by monitoring competitor activities that would require dedicated analyst teams. Avoid AI applications that require significant workflow changes or new regulatory frameworks—start with performance improvements to existing processes.
Author: William Flaiz