Why 73% of Pharma Digital Transformations Fail (And How the 27% Succeed)
During my first three months at Novartis, I met individually with executives from 6 of our top 10 markets. The conversations followed the same pattern: each defended why their local website strategy was "different." Meeting after meeting, I heard the same frustrated explanations of unique regulatory requirements, local compliance needs, and cultural considerations.
Sound familiar?
After analyzing hundreds of pharma digital transformation initiatives—both as an executive and consultant—I've discovered that 73% fail not because of technical challenges, but because they ignore a fundamental truth about pharmaceutical organizations.
They try to transform technology instead of transforming how people work with technology.

The Hidden Pattern Behind Digital Transformation Failure
Most pharma companies approach digital transformation backwards. They select platforms first, then force organizational behavior to adapt. This approach crashes against the complex web of stakeholders that make pharma unique: regulatory affairs, medical affairs, legal, compliance, commercial teams, and patient services.
Each group operates with different priorities, timelines, and success metrics. Legal focuses on risk mitigation. Commercial pushes for speed to market. Regulatory demands bulletproof compliance. Medical affairs requires scientific accuracy.
Traditional technology implementations treat these as obstacles to overcome rather than stakeholders to orchestrate.
The 27% that succeed flip this equation. They design transformation around stakeholder orchestration first, technology selection second.
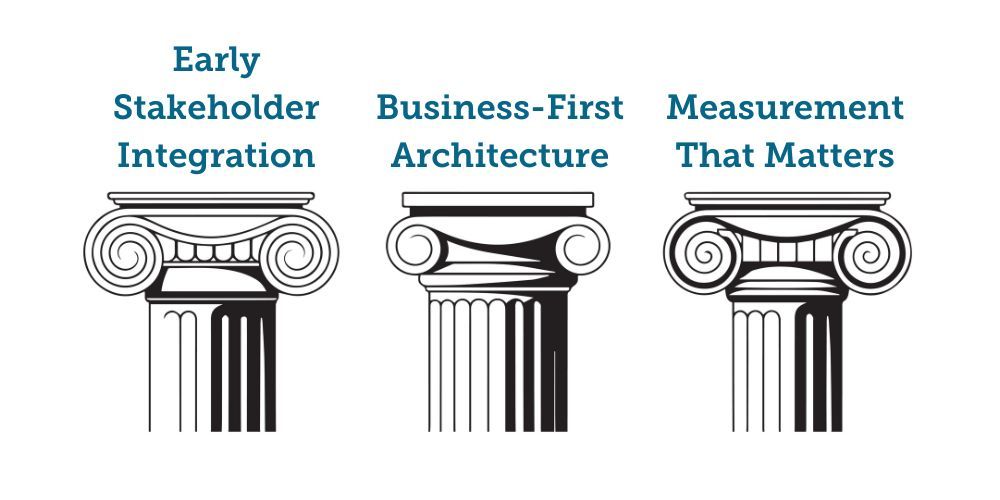
Framework: The Three-Pillar Success Model
After consolidating 1,200+ websites across 90 countries and preventing millions in compliance violations, I developed a framework that works regardless of company size or technological complexity.
Pillar 1: Early Stakeholder Integration
Most digital transformations bring legal and regulatory teams into conversations too late. They review final proposals, discover deal-breaking issues, and force expensive redesigns.
Smart organizations engage these stakeholders from day one.
At Novartis, I established bi-weekly cross-functional reviews before we wrote a single line of code. Legal, regulatory, compliance, medical affairs, and patient services participated in strategy development rather than strategy review.
This shift prevented the late-stage surprises that derail most implementations.
Implementation Steps:
- Map all stakeholder groups who could impact your project
- Schedule weekly check-ins during planning phases
- Create shared success metrics across departments
- Design feedback loops that surface concerns early
The investment in meeting time saves months of rework and millions in compliance costs.
Pillar 2: Business-First Architecture
Pharma companies often inherit technology architectures designed for speed rather than compliance. They bolt regulatory requirements onto platforms that weren't built for them.
Successful transformations architect solutions around business requirements first.
When designing our global web platform, we started with the most restrictive regulatory environment and built outward. Instead of creating 90 different compliance approaches, we designed one framework flexible enough to handle regional variations.
This approach delivered a 52% reduction in operating costs while ensuring bulletproof compliance across all markets.
Architecture Principles:
- Start with your most restrictive requirements
- Build flexibility through configuration, not customization
- Design for compliance validation at every step
- Plan for regional adaptation without core platform changes
Your technology should enforce compliance by design, not through manual oversight.
Pillar 3: Measurement That Matters
Traditional IT metrics focus on system performance: uptime, speed, user adoption. Pharma digital transformations require business outcome measurement.
The most successful initiatives track metrics that matter to business stakeholders:
- Time from content creation to regulatory approval
- Cost per market for content localization
- Risk exposure from non-compliant digital properties
- Revenue impact from improved market speed
These metrics create shared accountability between technology and business teams.
Case Study: Novartis Global Web Transformation
When I joined Novartis, they operated 1,200+ websites across 90 countries. Many sites were unmanaged, creating significant legal and regulatory exposure.
Instead of approaching this as a technology consolidation project, we treated it as a business risk mitigation initiative.
The Challenge
- Websites managed by different teams with varying compliance understanding
- No central oversight of content or regulatory requirements
- Significant exposure to legal action from non-compliant properties
- Massive operational costs from maintaining fragmented systems
Our Approach
We implemented the three-pillar framework:
- Early Stakeholder Integration: Legal and regulatory teams helped design the evaluation criteria for website criticality
- Business-First Architecture: We built retirement plans around regulatory risk rather than technical complexity
- Measurement That Matters: Success metrics focused on risk reduction and operational efficiency
Results
- Systematic retirement of 900 non-critical sites
- 52% reduction in operating costs
- Elimination of major regulatory exposure
- Framework that continued implementation after my departure
The final point matters most. Sustainable transformation survives leadership changes because it embeds change into organizational behavior rather than depending on individual champions.
The AI Strategy Workshop: Bridging Vision and Implementation
Organizations struggle to translate AI potential into pharma-specific applications. My AI Strategy Workshop helps teams identify practical AI implementations that align with regulatory requirements and business goals.
The workshop covers
- AI readiness assessment for pharma environments
- Compliance-first AI implementation strategies
- Stakeholder orchestration for AI adoption
- Measurement frameworks for AI business value
Recent Workshop Results
- 67% faster regulatory approval processes through AI-powered content analysis
- 45% reduction in compliance review cycles using automated validation
- 31% improvement in cross-market content localization speed
Why Most Organizations Skip These Steps
The three-pillar framework requires patience. Stakeholder integration extends planning timelines. Business-first architecture delays platform selection. Proper measurement takes time to implement.
Organizations under pressure choose speed over sustainability. They select platforms quickly, minimize stakeholder involvement, and measure technical rather than business metrics.
This approach feels faster initially but creates expensive problems later.
Regulatory violations, compliance gaps, and stakeholder resistance force costly revisions that eliminate any time savings from rushed implementation.
Your Next Steps
Successful pharma digital transformation requires discipline to prioritize process over technology.
Start by auditing your current approach:
- Are regulatory and compliance stakeholders involved in planning or just approval?
- Does your architecture enforce compliance by design or depend on manual oversight?
- Do your success metrics align with business outcomes or technical performance?
If you answered "approval," "manual oversight," and "technical performance," you're following the 73% failure pattern.
The 27% that succeed invest time upfront to avoid expensive corrections later.
Transform how people work with technology, and technology transformation follows naturally.
Should our company abandon traditional digital transformation approaches entirely?
Not necessarily. The decision depends on your stakeholder complexity and regulatory requirements. First, evaluate whether your current approach integrates compliance and regulatory teams during planning or only during review phases. If they're involved early and your projects consistently deliver on time and budget, your approach may work. However, if you experience frequent late-stage compliance surprises and cost overruns, adopting the three-pillar framework will provide better outcomes.
How long does the stakeholder integration process typically add to project timelines?
Early stakeholder integration adds 2-4 weeks to planning phases but typically reduces total project time by 8-12 weeks by preventing late-stage redesigns. At Novartis, bi-weekly cross-functional reviews during planning extended our initial timeline by 3 weeks but prevented a 16-week compliance remediation cycle that similar projects experienced. The upfront investment in stakeholder orchestration creates significant time savings during implementation and launch phases.
What specific skills should our teams develop for AI implementation in pharma environments?
Focus on developing regulatory-aware AI literacy rather than general AI skills. Teams need to understand how AI decisions can be validated for compliance, how to document AI-driven processes for regulatory review, and how to implement AI solutions that enhance rather than replace human oversight. Additionally, develop skills in cross-functional communication to help AI teams collaborate effectively with legal, regulatory, and medical affairs stakeholders who will ultimately validate AI implementations.
Author: William Flaiz