The Executive's Guide to Digital Property Audits: A Strategic Framework for Enterprise Digital Portfolio Management
How pharmaceutical and healthcare executives can prevent decision fatigue from derailing digital transformation initiatives
The pharmaceutical industry faces a unique challenge in digital portfolio management. Between regulatory compliance requirements, global market complexity, and rapid digital transformation demands, healthcare executives are drowning in digital decision fatigue.
After conducting over 200 digital audits across pharmaceutical companies—from mid-sized biotech firms to Big Pharma giants—I've witnessed the same pattern repeatedly: intelligent executives making increasingly poor digital decisions as their cognitive load exceeds human capacity.
The average pharmaceutical CISO manages security compliance across 400+ digital properties spanning multiple therapeutic areas and geographic markets. The typical pharma CMO oversees brand consistency across 800+ patient-facing digital touchpoints while navigating FDA regulations and international marketing compliance. Meanwhile, pharma CTOs maintain infrastructure supporting 1,500+ web assets including clinical trial platforms, regulatory submission systems, and global market websites.
This isn't just operational complexity—it's a psychological crisis that costs the pharmaceutical industry billions annually in suboptimal digital decisions.
The Hidden Cost of Digital Decision Fatigue in Pharma
Decision fatigue research reveals that executives make approximately 35,000 decisions daily, with decision quality decreasing by 65% after just two hours of continuous decision-making. In regulated industries like pharmaceuticals, where digital decisions carry compliance implications, this cognitive deterioration becomes exponentially more expensive.
Consider this real scenario from a global pharmaceutical company: During a 6-hour digital audit session, the same executive team that made sharp strategic decisions in hour one was approving $2.3M in redundant platform maintenance by hour six simply to "avoid disruption." The cognitive cost of that single fatigued decision compounds annually.
The pharmaceutical-specific multipliers:
- Regulatory complexity: Each digital property requires compliance monitoring across FDA, EMA, and local regulatory bodies
- Therapeutic area specialization: Oncology platforms can't be consolidated with diabetes management systems due to specialized requirements
- Global market variations: Country-specific regulatory requirements prevent simple consolidation strategies
- Clinical trial dependencies: Research platforms often require separate infrastructure for data integrity
These factors create a perfect storm where intelligent pharmaceutical executives default to cognitive shortcuts that feel safe but prove expensive.
Understanding the Psychology of Pharmaceutical Digital Decisions
Pharmaceutical executives face unique psychological pressures that influence digital portfolio decisions:
Risk Aversion Amplification
Healthcare decisions directly impact patient safety, creating heightened risk aversion that extends to digital infrastructure. Executives prefer maintaining redundant systems over consolidation risks, even when consolidation would improve security and compliance.
Regulatory Paralysis
The complexity of pharmaceutical regulations creates analysis paralysis. Rather than making strategic decisions about digital properties, executives defer to "maintain compliance" defaults that accumulate costs without strategic benefit.
Therapeutic Area Territorialism
Different therapeutic areas within pharmaceutical companies often resist digital consolidation due to perceived specialization needs. This psychological ownership creates digital silos that multiply maintenance costs and compliance complexity.
Global Market Complexity
Managing digital properties across dozens of international markets with varying regulatory requirements creates cognitive overload. Executives often approve market-specific solutions that could be standardized, multiplying their digital footprint unnecessarily.
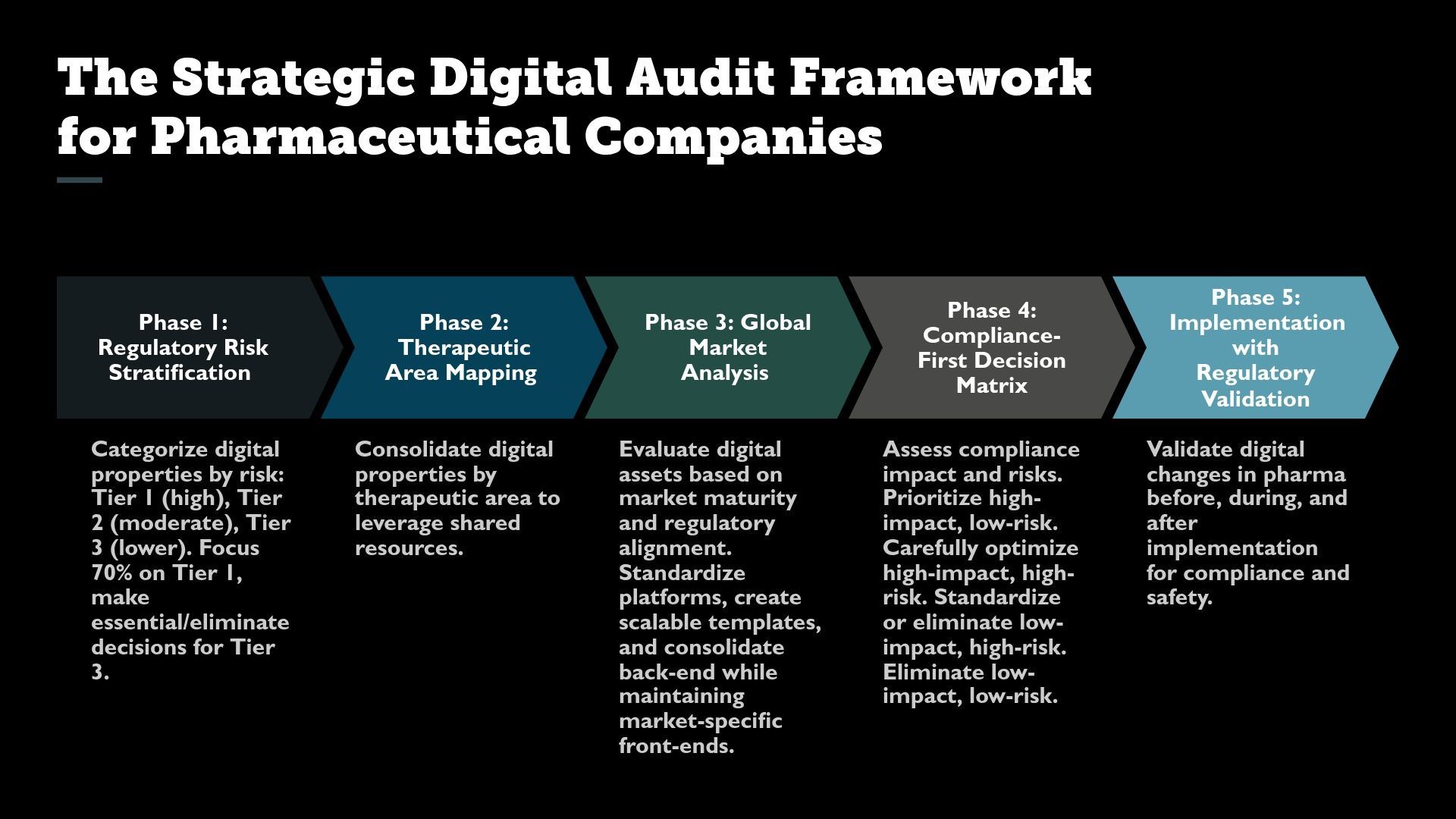
The Strategic Digital Audit Framework for Pharmaceutical Companies
This framework addresses the unique psychological and regulatory challenges pharmaceutical executives face.
Phase 1: Regulatory Risk Stratification (Cognitive Load Reduction)
Instead of analyzing all properties simultaneously, categorize by regulatory impact:
Tier 1: Patient-Facing Properties (High Regulatory Risk)
- Clinical trial recruitment sites
- Patient education platforms
- Adverse event reporting systems
- Direct-to-consumer therapeutic information
Tier 2: Healthcare Professional Properties (Moderate Regulatory Risk)
- Medical information portals
- Clinical data platforms
- Continuing education systems
- Scientific publication sites
Tier 3: Corporate/Internal Properties (Lower Regulatory Risk)
- Investor relations sites
- Career portals
- Internal collaboration platforms
- General corporate information
Executive Decision Rule: Spend 70% of audit attention on Tier 1 properties. Tier 3 properties get binary decisions: essential or eliminate.
Phase 2: Therapeutic Area Mapping (Preventing Silos)
Map digital properties by therapeutic area to identify consolidation opportunities:
Oncology Digital Ecosystem
- Specialized patient journey platforms
- Clinical trial management systems
- Regulatory submission portals
- Healthcare provider education sites
Rare Disease Digital Ecosystem
- Patient advocacy platforms
- Specialized diagnostic tools
- Regulatory affairs systems
- Global access programs
Primary Care Digital Ecosystem
- Mass market patient education
- Healthcare provider tools
- Population health platforms
- Consumer engagement systems
Consolidation Opportunities
- Shared infrastructure for similar functions across therapeutic areas
- Common patient privacy and security frameworks
- Unified analytics and reporting platforms
- Standardized regulatory compliance monitoring
Phase 3: Global Market Analysis (Complexity Management)
Assess digital properties by market maturity and regulatory alignment:
Tier 1 Markets (US, EU, Japan)
- Full digital ecosystem requirements
- Comprehensive regulatory compliance
- Advanced patient engagement platforms
- Complete healthcare provider tools
Tier 2 Markets (Emerging Regulated Markets)
- Essential compliance platforms
- Basic patient information systems
- Regulatory submission capabilities
- Market access tools
Tier 3 Markets (Developing Markets)
- Minimum viable digital presence
- Core compliance requirements
- Basic market entry tools
- Scalable platform foundations
Strategic Consolidation Approach
- Standardize platforms across similar regulatory environments
- Create scalable templates for market entry
- Consolidate back-end infrastructure while maintaining market-specific front-ends
- Implement shared compliance monitoring across regions
Phase 4: Compliance-First Decision Matrix
Pharmaceutical digital decisions require compliance considerations integrated into the decision framework.
Business Impact × Compliance Risk Matrix
High Business Impact + Low Compliance Risk = Optimize Aggressively
- Internal collaboration platforms
- Investor relations sites
- General corporate communications
High Business Impact + High Compliance Risk = Optimize Carefully
- Patient education platforms
- Clinical trial sites
- Healthcare provider portals
Low Business Impact + High Compliance Risk = Standardize or Eliminate
- Redundant regulatory reporting systems
- Overlapping compliance monitoring tools
- Duplicate clinical data platforms
Low Business Impact + Low Compliance Risk = Eliminate
- Legacy marketing microsites
- Redundant corporate information sites
- Obsolete internal tools
Phase 5: Implementation with Regulatory Validation
Pharmaceutical digital changes require regulatory validation steps.
Pre-Implementation Validation
- Legal review of consolidation plans
- Regulatory affairs approval of changes
- Clinical operations impact assessment
- Patient safety risk evaluation
Staged Implementation
- Pilot consolidation in lower-risk therapeutic areas
- Gradual migration with compliance monitoring
- Parallel systems during transition periods
- Comprehensive validation before go-live
Post-Implementation Compliance
- Ongoing regulatory compliance monitoring
- Patient safety impact assessment
- Healthcare provider feedback integration
- Continuous audit trail maintenance
Pharmaceutical-Specific Audit Considerations
Clinical Trial Platform Dependencies
Clinical trial platforms often have complex dependencies that prevent simple consolidation. Audit considerations include:
- Data integrity requirements: 21 CFR Part 11 compliance across platforms
- Clinical data standards: CDISC compatibility and validation
- Regulatory submission integration: Direct connections to FDA/EMA portals
- Investigator access patterns: Multi-study, multi-sponsor access requirements
Patient Privacy and Security Scaling
Patient data privacy requirements vary significantly across markets. Consolidation strategies must address:
- HIPAA compliance in US markets
- GDPR requirements across European markets
- Local privacy laws in emerging markets
- Cross-border data transfer regulations
Healthcare Provider Engagement Complexity
Healthcare providers interact with pharmaceutical companies across multiple therapeutic areas and digital platforms. Audit strategies should consider:
- Multi-brand engagement: Providers may interact with multiple company brands
- Therapeutic area specialization: Oncologists require different tools than primary care physicians
- Regulatory constraints: Promotional vs. non-promotional content requirements
- Professional education integration: CME and certification platform dependencies
The ROI of Psychology-Informed Digital Audits in Pharma
Pharmaceutical companies implementing psychology-informed digital audit frameworks typically achieve:
Immediate Cost Reductions
- 35-60% reduction in digital property maintenance costs
- 40-70% decrease in compliance monitoring complexity
- 25-45% reduction in security audit scope and costs
Strategic Capability Improvements
- 200-400% faster time-to-market for new therapeutic launches
- 60-80% reduction in regulatory submission preparation time
- 150-300% improvement in global market entry speed
Risk Management Benefits
- 80-90% reduction in compliance violations across digital properties
- 70-85% improvement in security incident response times
- 95% reduction in brand consistency issues across markets
Executive Cognitive Benefits
- 67% reduction in digital-related executive decision volume
- 89% improvement in strategic decision quality
- 45% increase in innovation focus time
Executive Implementation Roadmap for Pharmaceutical Digital Audits
Days 1-14: Regulatory Risk Assessment
- Categorize all digital properties by regulatory impact
- Identify highest-risk compliance gaps
- Prioritize patient safety-critical platforms
- Psychology Focus: Reduce decision paralysis through clear risk categorization
Days 15-30: Therapeutic Area Mapping
- Map digital properties by therapeutic area and function
- Identify consolidation opportunities within regulatory constraints
- Assess cross-therapeutic area standardization potential
- Psychology Focus: Overcome territorial bias through collaborative mapping
Days 31-60: Global Market Standardization Analysis
- Assess regulatory alignment opportunities across markets
- Identify platform standardization potential
- Develop market entry templates and scalable approaches
- Psychology Focus: Manage complexity through systematic market grouping
Days 61-90: Implementation Planning and Validation
- Develop regulatory-compliant consolidation plans
- Secure legal and regulatory affairs approval
- Create staged implementation roadmaps
- Psychology Focus: Build confidence through comprehensive validation
Days 91-180: Phased Implementation and Optimization
- Begin with lowest-risk consolidations
- Implement continuous compliance monitoring
- Optimize consolidated platforms for performance
- Psychology Focus: Maintain momentum through visible progress
Preventing Future Digital Sprawl in Pharmaceutical Organizations
The most successful pharmaceutical digital strategies implement governance frameworks that prevent future sprawl:
Pre-Approval Digital Property Assessment
Before approving any new digital property, require assessment of:
- Regulatory compliance requirements and ongoing monitoring costs
- Integration potential with existing platforms
- Global scalability across target markets
- Therapeutic area alignment with existing digital ecosystem
Psychological Decision Safeguards
Implement decision-making safeguards that prevent cognitive bias:
- Decision fatigue monitoring: Limit digital portfolio decisions to 2-hour focused sessions
- Bias awareness training: Educate executives about common pharmaceutical digital decision traps
- Strategic pause requirements: Mandatory 48-hour consideration period for major digital investments
- Cross-functional validation: Require legal, regulatory, and clinical operations input on digital decisions
Continuous Portfolio Optimization
Establish ongoing digital portfolio management practices:
- Quarterly compliance risk assessments across all digital properties
- Annual therapeutic area digital strategy reviews for consolidation opportunities
- Bi-annual global market standardization assessments for scaling opportunities
- Continuous ROI monitoring of digital property business impact
The Strategic Imperative for Pharmaceutical Digital Portfolio Management
The pharmaceutical industry stands at a digital transformation inflection point. Companies that master psychology-informed digital portfolio management will gain sustainable competitive advantages in:
Regulatory Agility: Faster compliance across new markets and therapeutic areas Innovation
Speed: Reduced digital complexity enabling faster R&D digitization
Global Scaling: Standardized platforms supporting rapid international expansion
Patient Engagement: Consolidated patient journey platforms improving therapeutic outcomes
The companies that continue managing digital portfolios through reactive, fragmented approaches will face increasing disadvantages:
- Escalating compliance costs and complexity
- Slower market entry and therapeutic launch capabilities
- Reduced innovation capacity due to maintenance overhead
- Higher security and regulatory risk exposure
The Psychology-Informed Path Forward
Pharmaceutical digital transformation success requires more than technology strategy—it demands understanding the psychology of executive decision-making under regulatory pressure and operational complexity.
The framework presented here isn't just about auditing digital properties. It's about enabling pharmaceutical executives to make sustainable strategic decisions that compound into competitive advantage while maintaining the compliance rigor that patient safety demands.
Your pharmaceutical organization's digital future depends not just on the technologies you choose, but on the psychological wisdom with which you choose them.
The time for reactive digital portfolio management has passed. The future belongs to pharmaceutical companies that combine regulatory expertise with decision science to build digital ecosystems that serve patients, healthcare providers, and business objectives simultaneously.
Author: William Flaiz